A limitation of the study on favipiravir was that it was not performed in a randomized double-blinded placebo-controlled clinical trial, allowing an inevitable selection bias in patient recruitment. However, given the high number of concurrent patients and the very high infectivity of disease, it was ethically impossible to condition patients to receive different experimental drugs using a randomization process that most patients would not be able to understand. It should be pointed out that the duration of favipiravir treatment in this clinical trial was twice as long as that used for influenza treatment. However, side effects in the experimental group were rare and well tolerated, and none of the patients needed to stop favipiravir treatment. These results seem to suggest that the duration of favipiravir treatment can be extended if necessary.
A recent study by Gao and colleagues using more than 100 patients showed that chloroquine phosphate had a better effect than controls on inhibiting pneumonia exacerbation and viral growth and shortening the course of the disease. However, this should be considered carefully as insufficient data are not available to support this result. The final interpretation therefore expects that in the absence of published data it is difficult to reach definite conclusions. It will be very important to know whether the observed efficacy is specifically related to chloroquine or hydroxychloroquine in their salt (phosphate or sulfate) form. Furthermore, it is also important to determine whether the benefits of chloroquine therapy depend on age classification or disease stage.
However, to get a complete clinical trial, it is not enough to just use the test on the patient, but you must get complete information regarding the side effects that are caused by the use of this drug. This phase is known as phase IV clinical trials (post marketing surveillance).
Potential therapeutic strategies against COVID-19
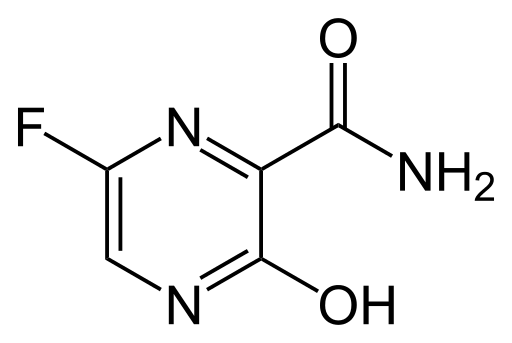
Initially, nebulized interferon-α, broad-spectrum antibiotics, and anti-viral drugs could be used to reduce viral load in the body, however, only remdesivir has shown a promising effect on viruses. Remdesivir alone and in combination with chloroquine or interferon beta were significantly able to inhibit SARS-CoV-2 replication so that patients were declared clinically cured. Other antiviral agents currently being evaluated against infection include Nafamostat, Nitazoxanide, Ribavirin, Penciclovir, Favipiravir, Ritonavir, AAK1, Baricitinib, and Arbidol showing satisfactory results when tested against infection in patients and in-vitro clinical isolates.
Recently in Shanghai, doctors isolated blood plasma from COVID-19 patients who had recovered clinically and injected it into infected patients. The results are positive with a fast recovery. In a recent study, it was identified that a monoclonal antibody (CR3022) is able to bind to the receptor binding site of SARS-CoV-2. CR3022 has the potential to be developed as a therapeutic candidate, either alone or in combination with other neutralizing antibodies for the prevention and treatment of COVID-19 infection.
Author: Dr. apt. Yandi Syukri, S.Si, M.Si. (Pharmacy Lecturer and Researcher at the Nanopharmacy Research Center, Department of Pharmacy, Islamic University of Indonesia)
Source:
[1] Y. Duan, H.-L. Zhu, C. Zhou, Drug Discov. Today 2020.
[2] A. Cortegiani, G. Ingoglia, M. Ippolito, A. Giarratano, S. Einav, J. Crit. Care 2020.
[3] F. Touret, X. de Lamballerie, Antiviral Res. 2020, 177, 104762.
[4] M. Adnan Shereen, S. Khan, A. Kazmi, N. Bashir, R. Siddique, J. Adv. Res. 2020.
[5] Q. Cai, M. Yang, D. Liu, J. Chen, D. Shu, J. Xia, X. Liao, Y. Gu, Q. Cai, Y. Yang, C. Shen, X. Li, L. Peng, D. Huang, J. Zhang, S. Zhang, F. Wang, J. Liu, L. Chen, S. Chen, Z. Wang, Z. Zhang, R. Cao, W. Zhong, Y. Liu, L. Liu, Engineering 2020.
[6] K. Shiraki, T. Daikoku, Pharmacol. Ther. 2020, 107512.
[7] C. A. Devaux, J.-M. Rolain, P. Colson, D. Raoult, Int. J. Antimicrob. Agents 2020, 105938.